
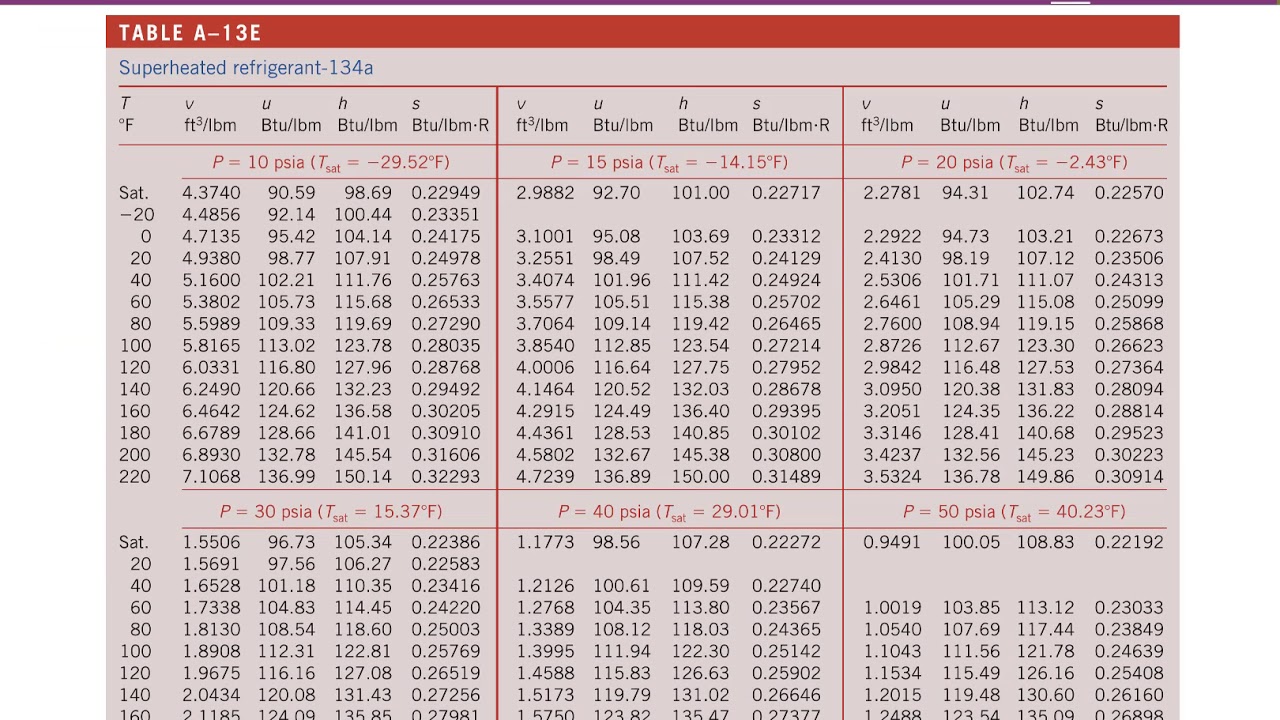
We would construct a conversion factor between the number of moles of H 2 and the energy given off, −184.6 kJ: Suppose we asked how much energy is given off when 8.22 mol of H 2 react. The equivalences for this thermochemical equation are: This is why the unit on the energy change is kJ, not kJ/mol.įor example, consider the following thermochemical equation: Thus, 2 mol of H 2 are related to −570 kJ, while 1 mol of O 2 is related to −570 kJ. When an amount of energy is listed for a balanced chemical reaction, what amount(s) of reactants or products does it refer to? The answer is that relates to the number of moles of the substance as indicated by its coefficient in the balanced chemical reaction. Note that these equivalences address a concern. This equivalence can also be used to construct conversion factors so that we can relate enthalpy change to amounts of substances reacted or produced. That is, we can now add an energy amount to the equivalences - the enthalpy change of a balanced chemical reaction. This new quantity allows us to add another equivalence to our list: Where ⇔ is the mathematical symbol for “is equivalent to.” In our thermochemical equation, however, we have another quantity - energy change: In this equation, we recognize the following equivalences: For example, observe the following balanced chemical equation:

In Chapter 5 “Stoichiometry and the Mole”, we related quantities of one substance to another in a chemical equation by performing calculations that used the balanced chemical equation the balanced chemical equation provided equivalences that we used to construct conversion factors.
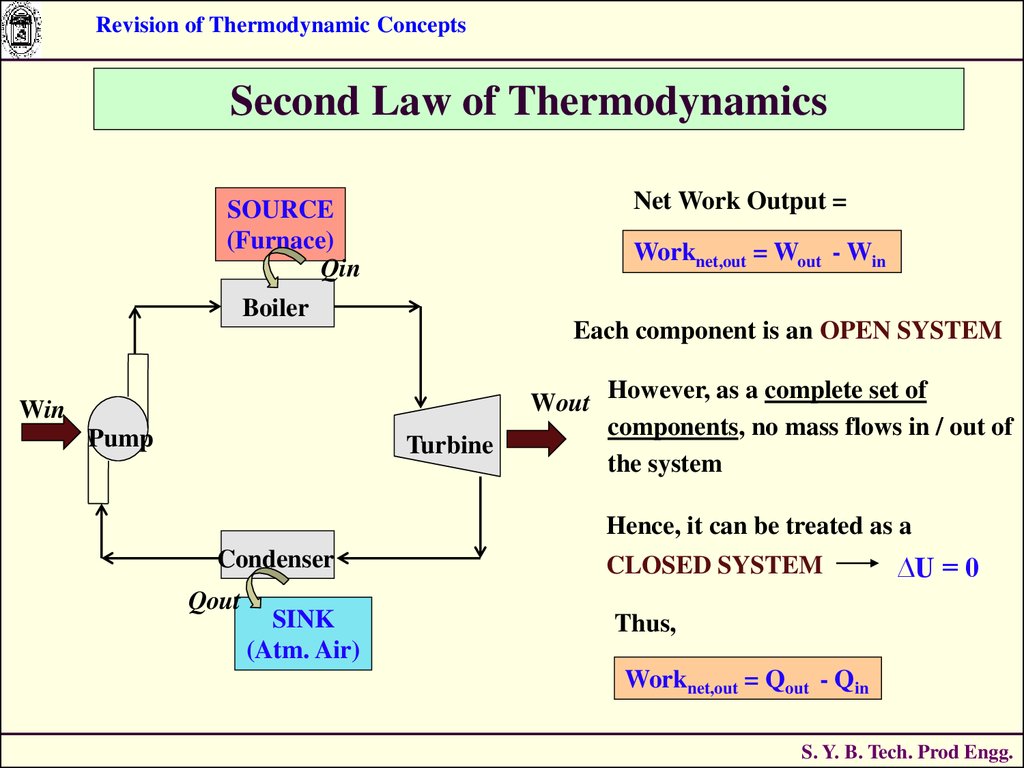
Perform stoichiometry calculations using energy changes from thermochemical equations.


 0 kommentar(er)
0 kommentar(er)
